1 in Your Own Words Describe Colligative Properties 1 Point
So for example if both calcium chloride CaCl 2 and sodium chloride NaCl completely dissolve in water the calcium chloride would lower the freezing point more. Colligative Properties Team No.
Sprinkling salt on icy roads to melt the ice is an example of Freezing Point Depression.
. Express your answer in both K and C. Worlds Best PowerPoint Templates - CrystalGraphics offers more PowerPoint templates than anyone else in the world with over 4 million to choose from. In your own words describe colligative properties.
In your own words briefly state the purpose of the lab. You never measure osmolarity in practice because water changes its volume according to temperature but mass remains the same and so it is more convenient and consistent. Osmolality is the measure of solute concentration per unit mass of solvent.
State the purpose of Part 1 and describeexplain the method. Describe in your own. Osmolarity is the measure of solute concentration per unit volume of solution.
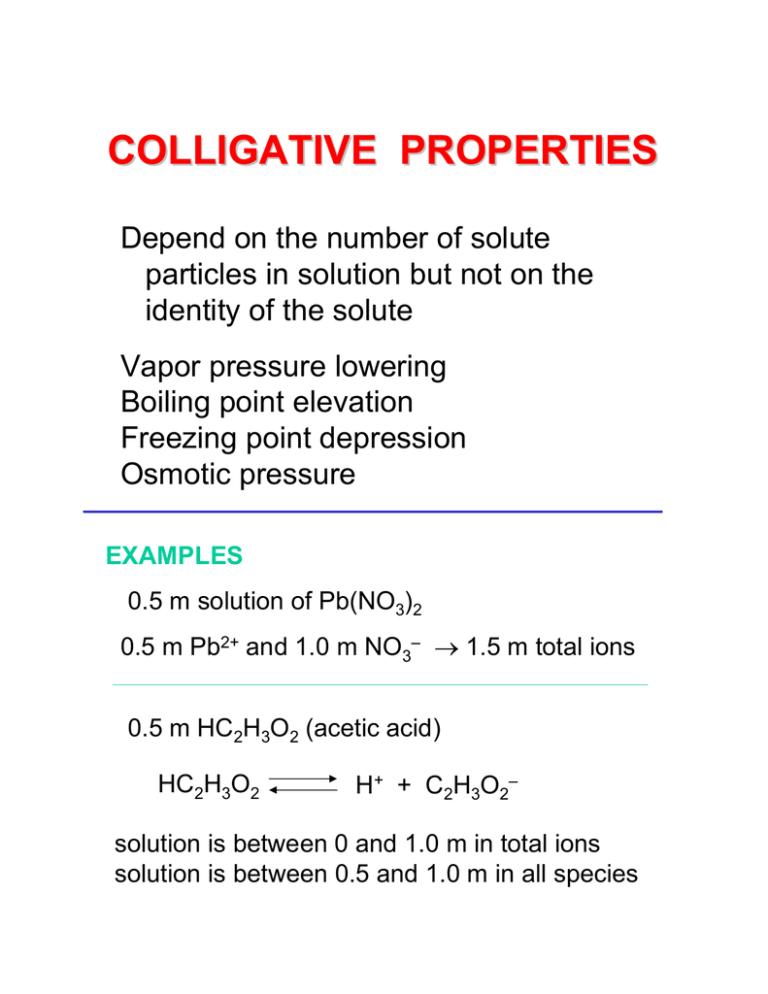
We are accustomed to describing a solution in terms of the. 1 point Colligative properties are properties of solutions that depend on the number of particles in a volume of solvent. Colligative properties depend on the number of particles present not on the type of particles or their mass.
Handwritten or typed answers are acceptable. Freezing Point Data Use a pen to record all results Solved. Colligative Properties Team No.
Recall that colligative properties predict that impurities lower meltingfreezing points. Freezing point depression is a colligative property of matter. Colligative properties depend only on the amount and not the nature of the solute dissolved in the solvent.
3 - Freezing point depression. Vapor pressure lowering freezing point depression boiling point elevation osmotic pressure. Up to 24 cash back -1 Discussion.
Colligative Properties Post lab Questions 15 points 1. I Relative lowering in vapour pressure ii Elevation in boiling point iii Depression in freezing point iv Osmotic. 4 - Role of phase diagrams.
A detailed explanation one paragraph or more in your own words of the colligative property being discussed and why that property changes the way that it does when the amount of solute is increased. The properties of a solution differ from those of a pure solvent due to interactions that take place between the solute and solvent molecules. Recrystallization of acetanilide from water1 The purity of the crude and recrystallized acetanilide will be assessed by melting point.
In your own words briefly state the purpose of the lab. List the freezing point depression and boiling point elevation equations there are total of 4. Vapor pressure depression ΔP P 0 χ mole fraction which is the amount of solute added depression constant which is the vapor pressure of pure solvent at a.
Use only your own words Checklist 45 pts max for including the items listed below in your report Overall introduction to experiment 025 pt Part 1. Name three colligative properties discussed in the text. One way to understand why the freezing point of a mixture is lower than that of the pure solvent is to.
The colligative properties of a solution depend only on the number of solute particles dissolved True Given that the chemical formula of Lauric acid is CH3CH210COOH and the atomic weights of C H and O are 12 gmol 1 gmol and 16 gmol respectively calculate the molecular weight of this compound. Kaylyn Kirk Week 6. The normal boiling point of water is 373 K so we have a ΔTb 25 K which equates to an effective molality of m 5 resulting in a ΔTf -1 K so our solution will freeze at 272 K or -1 C.
5 - Colligative properties and entropy. 1 point Colligative properties are solution properties that only depend on the concentration of a solute and disregards their identities. Freezing Point Data Use a pen to record all results Experiment Freezing Point C Tf C Moles of Solute.
Colligative properties are properties of solutions that depend on the number of particles in a volume of solvent the concentration and not on the mass or identity of the solute particles. 2 - Boiling point elevation. Colligative Properties Team No.
Crude acetanilide from water Scheme 1. Winner of the Standing Ovation Award for Best PowerPoint Templates from Presentations Magazine. Write the chemical equations showing the dissolution of sodium chloride naphthalene potassium phosphate and calcium chloride.
Colligative properties are also affected by temperature. Colligative Properties of Matter. What you should be able to do.
Theyll give your presentations a professional memorable appearance - the kind of sophisticated look that. Calculation of the properties only works perfectly for ideal solutions. 1 - Raoults law and vapor pressure.
Called Freezing Point Depression and is a colligative property of solutions. The freezing point of a solution that boiled at 3755 K. 9 pts _____ Write the discussion in paragraph form not in outline form.
This document will print in an appropriately modified format 12 pages. Your article should include the following. In your own words describe colligative properties.
5 pts per question 1. The properties that exhibit such changes are called the colligative properties and include vapor pressure lowering boiling point elevation freezing point depression and changes in osmotic pressure see. Experiment 15 Colligative Properties Postlab.
Colligative Properties Chemistry Class 12 Solutions. Instructor Answer the following questions using complete sentences and support your answers with calculations if desired. This small set of properties is of central importance to many natural phenomena and technological applications as will be described in this module.
List the freezing point depression and boiling point elevation equations there are total of 4. Properties of a solution that depend on the number of particles present rather than on the identity or properties of the particles any three of the following. These colligative properties include vapor pressure lowering boiling point elevation freezing point depression and osmotic pressure.
Questions 20 points possible Name Section daytime Due date.

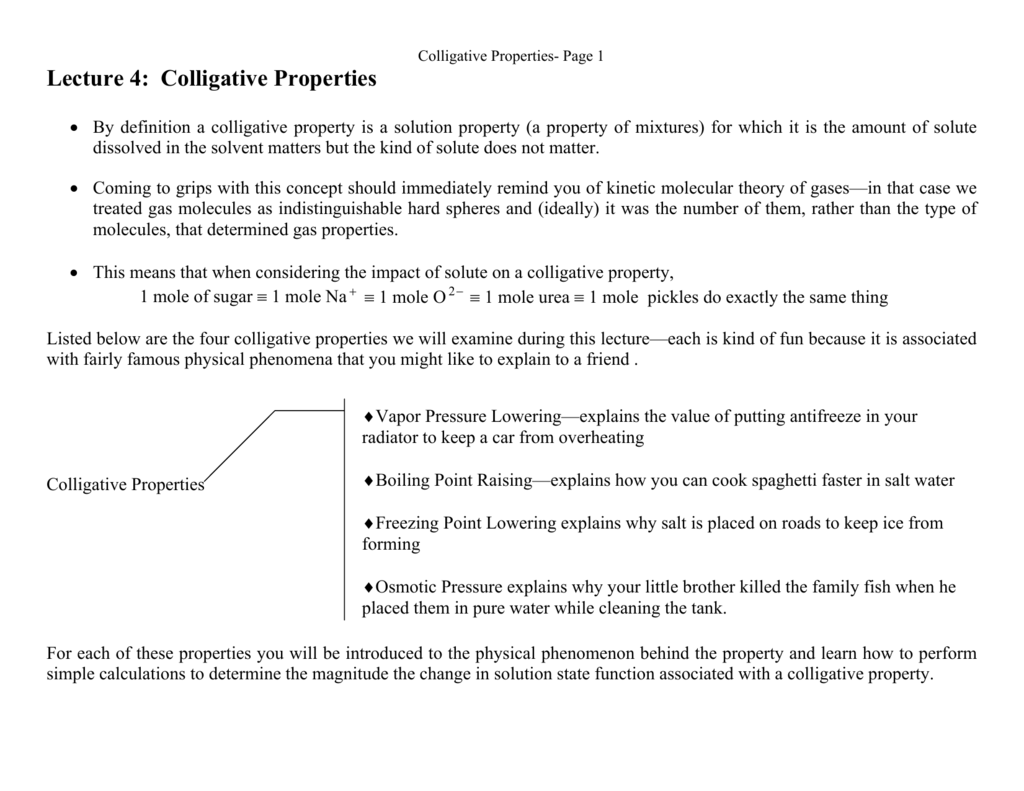
Lecture 4 Colligative Properties

Belum ada Komentar untuk "1 in Your Own Words Describe Colligative Properties 1 Point"
Posting Komentar